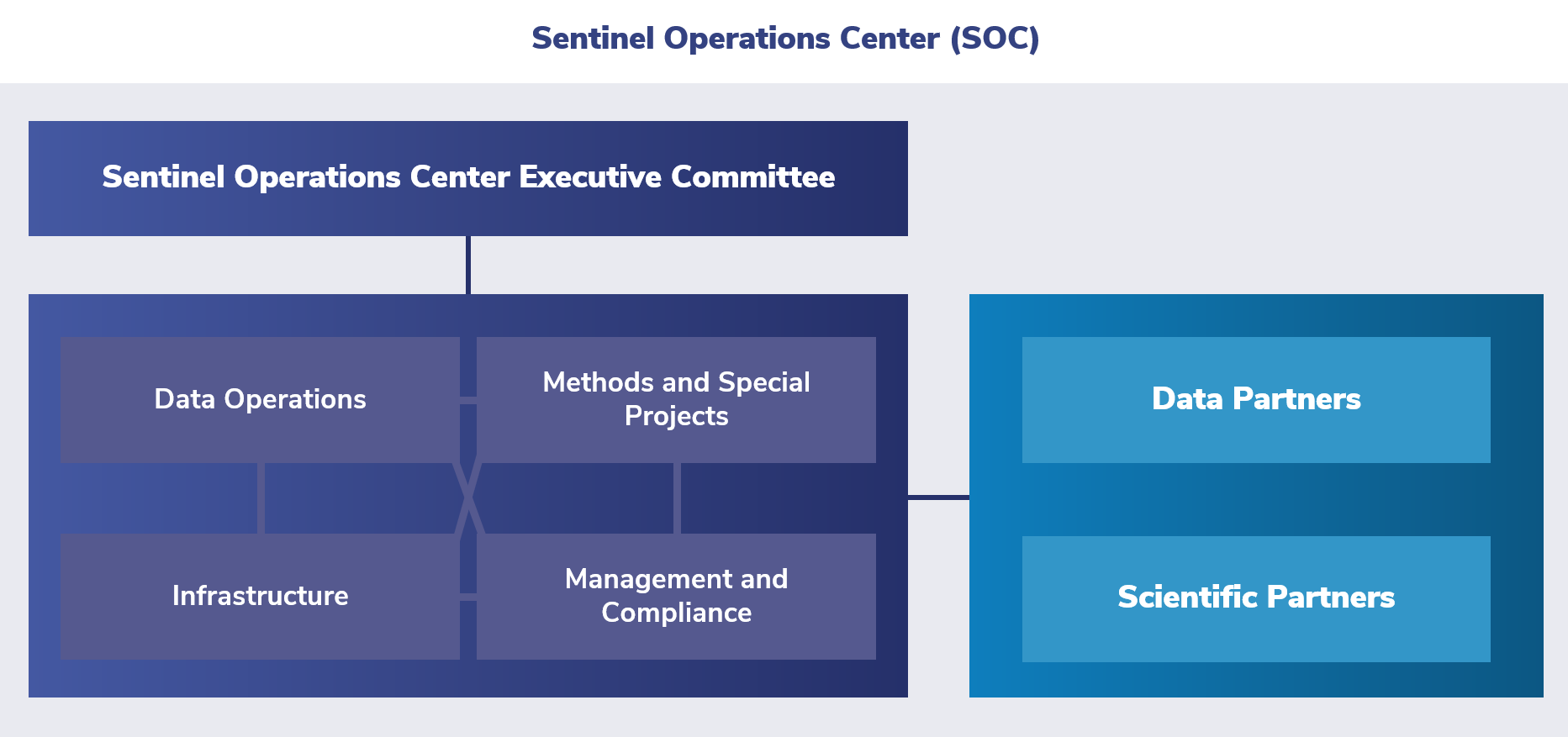
The Sentinel Operations Center resides within the Harvard Pilgrim Health Care Institute. The Sentinel Operations Center works with the FDA to assess the risks and benefits of marketed medical products.
Leadership





Professional headshots by Elisif Photography
Objective
Harvard Pilgrim Health Care Institute leads the Sentinel Operations Center in collaboration with Sentinel’s Data Partners, using a distributed database. This center leverages organizational partnerships in the areas of:
- Epidemiology
- Clinical medicine
- Pharmacy
- Statistics
- Health informatics
- Data science (specifically, artificial intelligence tools like natural language processing and machine learning)
- Network operations to support post market safety analyses
The primary functions of the Sentinel Operations Center are to:
- Enhance core data sources and evolve the Sentinel Common Data Model
- Develop novel distributed analytic tools
- Further enhance capabilities in signal detection and other areas
- Lead Real World Evidence demonstration projects through FDA-Catalyst
How the Sentinel Operations Center Assesses the Risks and Benefits of Marketed Medical Products
Below is how the Sentinel Operations Center answers the FDA's questions on marketed medical products
1. The Sentinel Operations Center works with the FDA to design studies that can get answers to the FDA’s questions.
The Sentinel Operations Center creates computer programs to conduct the studies. These programs use medical billing information and electronic health records from Data Partners.
2. The Sentinel Operations Center sends these programs to the Data Partners, who run the programs on their own data.
Data Partners return the study results to the Sentinel Operations Center. They make sure to do this without revealing personal information from any patients.